
【引言】
金属有机骨架(MOF)是以金属离子或金属簇为节点,有机分子为连接体形成的一类多孔材料,可以用不同大小和形状的三维网络来表示。由于其独特的优势(如高表面积、孔隙度、柔韧性和可调的多孔结构),MOF在许多领域都有很好的应用前景,这些领域包括气体储存和分离、多相催化、传感器技术、药物输送、能量储存等。到目前为止,MOF已经成为一个快速发展的研究领域,已开发出的MOF超过2万种。镧系MOF(Ln-MOF)作为MOF材料的一种,由于其发光量子产率高、Stokes位移大、发射寿命长、发射带窄、耐光漂白等独特的发光性能而备受关注。在制备方法上,通常采用溶剂/水热法合成纯MOF材料。例如,作为最有趣的Ln-MOF之一,MOF-76是由三价镧系离子与1,3,5-苯三羧酸(H3BTC)在N,N´-二甲基甲酰胺(DMF)和去离子水的混合物中温和温度(高达80℃)下制备的。遗憾的是,这种合成策略导致大多数刚合成的 Ln-MOF稳定性差,发射强度有限,严重制约了其在各个领域的实际应用。因此,合成稳定性显著提高且高发光的MOF材料是非常需要的。
众所周知,很多的无机材料也采用溶剂/水热反应制备,尽管制备后的材料性能不理想,但这些材料经高温退火处理后性能却得到显著改善。然而,如果采用水热法合成MOF-76(Gd),然后再800℃煅烧4h,则只能得到替代α-Gd2O3纳米棒。发生这种现象的原因是MOF中的有机成分被焚烧殆尽,也就意味着利用高温退火获得高性能的MOF材料是不可行的。但是,一个不可忽略的事实是上述高温退火是在空气气氛中进行的。那么,如果将MOF置于高压环境中,能否在相对较低的温度下达到退火效果?最近,孟等人提出MOF-76是Tb3+离子的优秀宿主,但对Eu3+的发射却很不友善,即使采用Tb3+/Eu3+共掺杂体系Eu3+的发光依旧较弱。
为了解决上述问题,南昌工程学院桂卫军、万利佳和斯洛伐克共和国M. Almáši合作提出了用于MOF退火的普适方法-水热退火法,制备稳定性显著增强、高发光的MOF-76(Gd,Tb, Eu)等。也就是先在80 oC水热合成MOF-76,然后将之140 oC水热反应4小时。有趣的是,经过退火处理后,MOF-76从原来的四方相转变为单斜晶相,并表现出以下新颖的性质:1. 退火处理使发光强度提高2倍,光致发光量子产率(PLQY)由10.86 %提高到61.48 %(个人认为产率测试结果偏低,因为得到的MOF很亮);2. 首次观察到MOF-76中Eu3+离子的5D0→7F1跃迁明显增强;3. 由于退火,棒状晶体聚集形成片状结构;4. 退火后的MOF具有较强的抗水分子猝灭性,以及较好的紫外、化学和热稳定性。可以预见,该材料具有显著增强和高度稳定的发光特性,将为其提供新的应用前景。此外,MOF-76可以在各种有机溶剂中退火,这些溶剂包括乙二醇、二氯甲烷、丙酮以及乙醇等。成功实现Ln-MOF的水/溶剂热退火表明本文提出的方法为显著提高其他MOF的性能开辟了新途径,是一种普适方法。最后,将获得的绿色和红色发光的aMOFs应用于暖白光LED。
【结果】
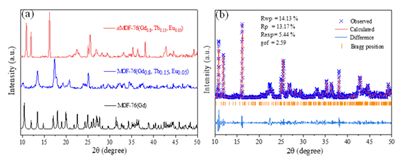
Fig. 1 Powder X-ray diffraction patterns of MOF-76(Gd0.8,Tb0.15,Eu0.05) and its annealed counterpart(a); Rietveld refinements of XRD data for aMOF-76(Gd0.8,Tb0.15,Eu0.05).
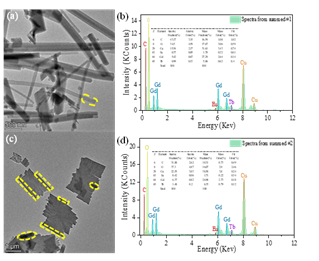
Fig. 2 TEM image(a) and energy dispersive X-ray (EDX) spectra(b) of the as-prepared MOF-76 (Gd0.8,Tb0.15,Eu0.05); TEM image(c) and EDX spectra(d) of the annealed sample aMOF-76 (Gd0.8,Tb0.15,Eu0.05).
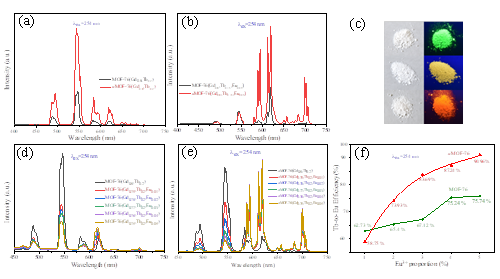
Fig. 3 Emission spectra of the AP MOF-76(Gd0.8,Tb0.2) and its annealed counterpart (a); emission spectra of the AP MOF-76(Gd0.8,Tb0.15,Eu0.05) and its annealed counterpart (b); photographs of the annealed MOF-76(Gd0.8,Tb0.2), AP MOF-76(Gd0.8,Tb0.15,Eu0.05) and its annealed counterpart under daylight and 254 UV lamp, respectively (c); emission spectra of the AP MOF-76(Gd0.8-x,Tb0.2,Eux) (d) and its annealed counterpart (e), with x=0, 0.01, 0.02, 0.03, 0.04 and 0.05; energy transfer efficiency from Tb3+ to Eu3+ in the AP MOF-76(Gd0.8-x,Tb0.2,Eux) and its annealed counterpart (f).
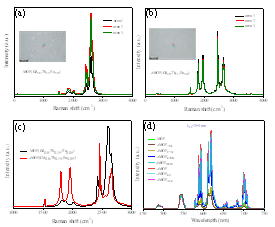
Fig. 4 Raman spectra of MOF(Gd0.8,Tb0.15,Eu0.05) (a) and aMOF(Gd0.8,Tb0.15,Eu0.05) (b), where the insets show the measured regions within the materials; Raman spectra of AP MOF and aMOF compounds used for mutual comparison (c); emission spectra of the as-prepared MOF(Gd0.8, Tb0.15, Eu0.05), aMOF(Gd0.8, Tb0.15, Eu0.05) and the MOF(Gd0.8, Tb0.15, Eu0.05) annealed at 140 oC for 4 h in various solvents, including toluene (TOL), cyclohexane (CYH), ethyl alcohol (ETOH), dichloromethane (DCM), ethylene glycol (EG) and acetone (ACE) (λex =254 nm).
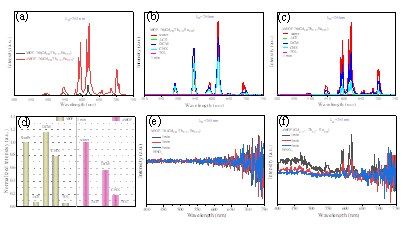
Fig. 5 Emission spectra of MOF-76(Gd0.8, Tb0.15, Eu0.05) and its annealed counterpart when dispersed in water (a); emission spectra of MOF-76(Gd0.8, Tb0.15, Eu0.05) (b) and its annealed counterpart (c) after soaking in the solvents (water, ACE, DCM, CHX and TOL) for 1 minute; emission intensities of MOF-76(Gd0.8, Tb0.15, Eu0.05) and its annealed counterpart in different solvents after 1-minute soaking (d); emission spectra of MOF-76(Gd0.8, Tb0.15, Eu0.05) (e) and its annealed counterpart (f) after soaking them in the mixture consisting of nitric acid and water for 1, 3 and 5 minutes, respectively.

Fig. 6 Emission spectra of the AP MOF(Gd0.8, Tb0.15, Eu0.05) (a) and its annealed counterpart (b) separately dispersed in a mixture consisting of ammonia and water, with the volume ratio varying from 0 to 0.5; the relationship between emission intensity and volume ratio of the two MOFs (c); the relationship between the intensity of the two MOFs and soaking time (d); the relationship between the intensity of the two MOFs and ultraviolet irradiation time (e); the relationship between the intensity of the two MOFs and heat irradiation time.

Fig. 7 Emission spectra of the red LED based on aMOF(Gd0.8, Tb0.15, Eu0.05) (a); emission spectra of the green LED based on aMOF(Gd0.8, Tb0.2) (b); emission spectra of the white LED based on the combination of aMOF(Gd0.8, Tb0.15, Eu0.05), aMOF(Gd0.8, Tb0.2) and N-CDs@starch (c); the corresponding CIE chromaticity coordinates of red, green and white LEDs. Insets of figures (a), (b) and (c) are photographs of the corresponding LEDs.
【原文链接】
Weijun Gui, Mingzhe Du, Xianmin Zhang, Miroslav Almáši, Lijia Wan, Lu Gan, Zhenxi Wang, Suhang Wu. A strategy to obtain highly luminescent MOF-76 based on hydrothermal annealing treatment, Journal of Alloys and Compounds, 2025, DOI: 10.1016/j.jallcom.2025.179341
